Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
Last month, the U.S. Centers for Disease Control and Prevention (CDC) made an important change to its COVID-19 vaccine recommendations. Under the new guidance, people who are immunocompromised and received a two-dose mRNA COVID-19 vaccine (Pfizer or Moderna) can now get an additional third dose as part of the vaccine series.
Research has shown that immunocompromised people — from organ transplant recipients to people with inflammatory or autoimmune conditions who take immunosuppressant medication — may not garner the same immune response to the two-dose vaccine series as non-immunocompromised people.
The additional dose is “intended to improve immunocompromised people’s response to their initial vaccine series,” according to the CDC.
However, the degree to which the immune response is affected can vary widely depending on the medications you take and your underlying health issues. Remember: The original two-dose COVID-19 vaccine series is providing some protection for many immunocompromised people; the third dose will help make that protection even stronger.
The recommendation for the third COVID-19 vaccine dose comes as COVID-19 case numbers have been on the rise for weeks across the U.S., due to the spread of the Delta variant and an increase in breakthrough cases.
Anecdotally, many members of the CreakyJoints and the Global Healthy Living Foundation have been anxiously waiting for an additional COVID-19 vaccine dose to be approved. We conducted a poll of our COVID-19 Patient Support program to gain insight into whether our members, who are immunocompromised and high-risk for COVID-19, have received or plan to receive their third COVID-19 vaccine dose.
The poll was conducted from August 26 to 30, 2021, about two weeks after the third dose became available to immunocompromised people.
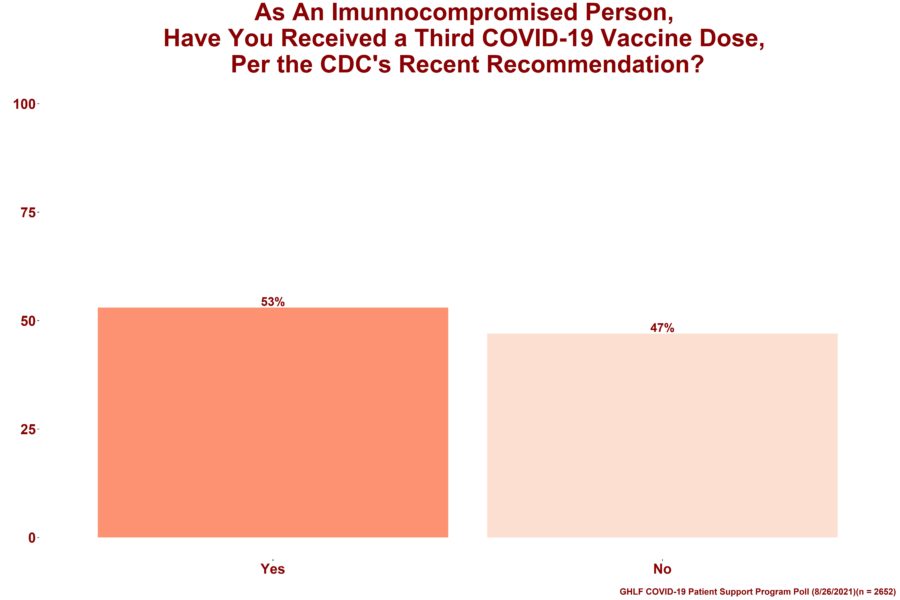
Among 2,738 respondents (97 percent are fully vaccinated), more than half (53 percent) said they had already received the third dose.
Here’s more about what we learned.
Most immunocompromised people who have not gotten their third COVID-19 vaccine dose plan to.
Of the poll respondents who said they have not yet received their third COVID-19 vaccine dose, 91 percent plan to do so. Some people said they are waiting to speak with their doctor or need to time the vaccine around their medication regimen.
(The American College of Rheumatology’s (ACR) COVID-19 Vaccine Guidance recommends that people on many immunosuppressant and immunomodulating medications temporarily stop them for one to two weeks after getting the third dose to help improve their immune system response.)
“I am getting the third dose as soon as possible. I have to time it with my medications though which is why I did not get it immediately,” one respondent explained.
“I’m grateful to be able to get an additional dose and plan to do so in the coming weeks but am waiting, as per my doctor’s instruction, to finish a treatment,” another person shared.
Read more here about the ACR’s guidance for timing specific medications to the third COVID-19 vaccine dose.
Several participants also noted that they haven’t received their additional COVID-19 dose because they received the single-dose Johnson & Johnson vaccine, for which an additional dose is not currently recommended. According to the CDC, “there is not enough data at this time to determine whether immunocompromised people who received the Johnson & Johnson’s Janssen COVID-19 vaccine also have an improved antibody response following an additional dose of the same vaccine.” CDC and FDA officials are actively studying this issue and expect to have an update for people who received the Johnson & Johnson vaccine soon.
Side effects after the third COVID-19 vaccine dose were common and mostly mild.
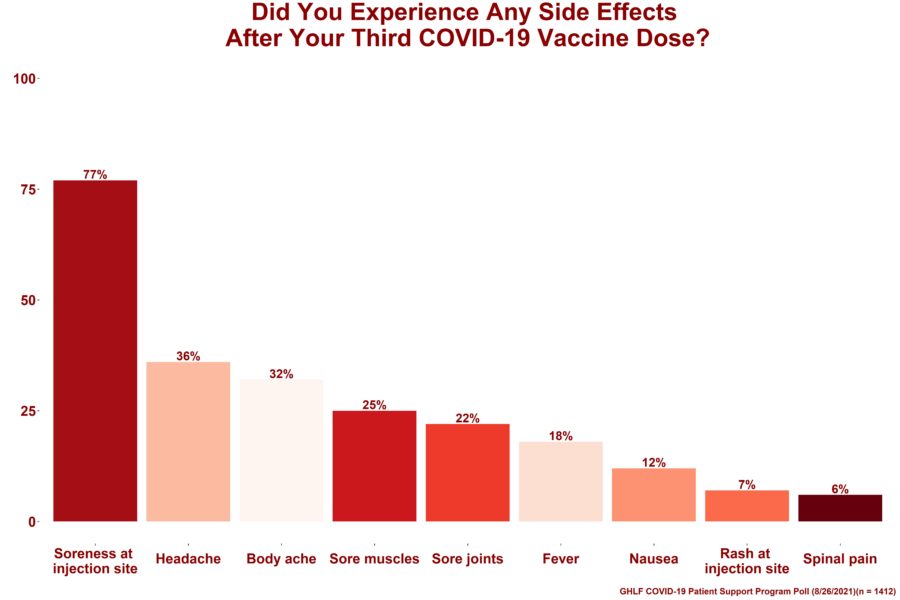
As part of the poll, people were asked to select the side effects they experienced after their third COVID-19 vaccine dose. The options were chosen based the CDC’s list of most common COVID-19 vaccine side effects, as well as those shared by GHLF and CreakyJoints community members. Of the 1,412 who answered:
- 77% had soreness at the injection site
- 36% had headache
- 32% had body aches
- 25% had sore muscles
- 22% had sore joints
- 18% had fever
- 12% had nausea
- 7% had a rash at the injection site
- 6% had spinal pain
- 12% had other side effects
- 11% had none of the previously listed side effects
Respondents were able to share more details about their side effects in a free response section. Many wrote that they dealt with chills, fatigue, and brain fog after receiving their third COVID-19 vaccine dose. Several also noted that they experienced malaise, a general feeling of unwellness that is similar to the flu. Five people wrote that they experienced some type of disease flare following the additional dose.
Read more here about what it was like for those in our community who received the third COVID-19 vaccine dose.
No one likes to feel discomfort or be out of commission for a few days. But many said they’d happily deal with the temporary side effects if it meant extra protection.
“Horrible side effects but definitely worth it,” one person wrote.
“I felt very run down for three days afterward, but enhanced protection against COVID is worth a couple of days of not feeling my best,” another shared.
Similarly, one person noted that they had a “rough 48 hours after receiving the injection, but it was worth it.” Another wrote that they were “miserable for about 12 hours, but that’s a trade-off I’ll take for the extra protection.”
Several people said the side effects were a sign that the vaccine is doing its job to build up protection.
“I definitely had a more pronounced reaction to the third dose than the previous two, but it was totally worth it and maybe just a sign that my immune system is working as it should,” one person wrote.
“[I experienced] congestion and fatigue, which I was glad about as it indicated that I did have some antibodies in place,” another patient shared.
“This is the first time I’ve had any side effects other than a sore arm at the injection site, so I’m hopeful that’s a good sign,” one person said.
Most people who received the third COVID-19 vaccine dose felt ‘hopeful’ and relieved’ afterward.
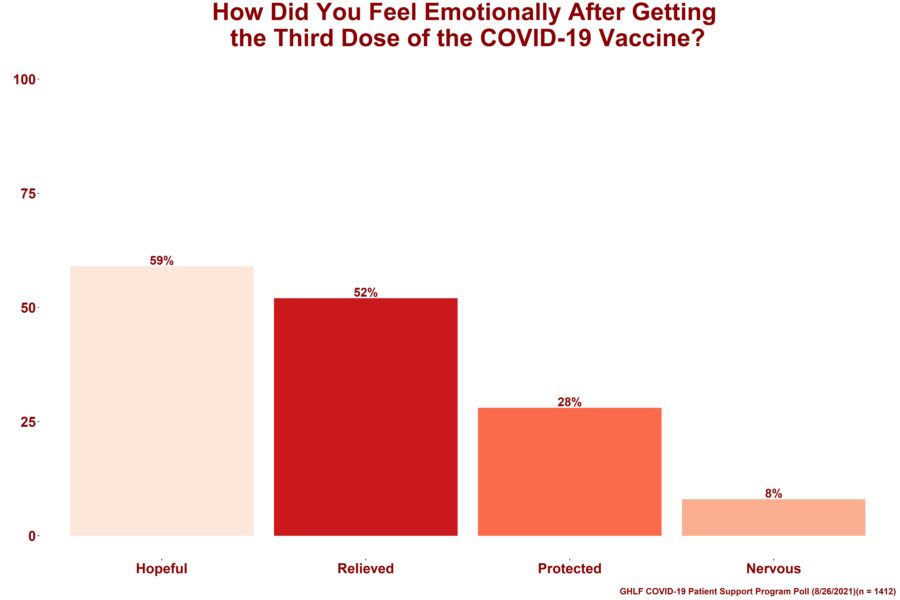
Respondents were also asked to choose all the emotions they felt after getting the third dose of the COVID-19 vaccine. Of the 1,412 who responded:
- 59% felt hopeful
- 52% felt relieved
- 28% felt protected
- 8% felt nervous
- 12% felt other emotions
- 3% felt none of the previously listed emotions
In the free response section where patients could elaborate on their answers, many wrote that their optimism comes with mixed emotions.
“I am hopeful that this third dose gives us more protection but also still nervous, scared, and stressed that I might not have gotten enough protection,” one person shared.
Another echoed these sentiments: “I am afraid to get my hopes up that this will protect me.”
Many patients noted that they would continue to exercise precautions to protect themselves from COVID-19. This is in line with the CDC’s messaging, which urges immunocompromised people to continue practice mitigation efforts, such as wearing a mask and social distancing, even after receiving their additional COVID-19 vaccine dose.
“While I am hopeful I will finally mount an immune response after the third shot it won’t make any difference in my behavior since the vaccinated people who are getting sick enough to be hospitalized or die are primarily immunocompromised,” one patient wrote.
“Although I am hopeful, I am still nervous and will continue to wear mask inside and sometimes out,” another person shared, adding they would continue to avoid eating in restaurants and being at venues with large groups of people.
“I feel hopeful, but I’m not sure why, because nothing will change for me,” another respondent wrote. “I’ll still be masked, and distanced, and working remotely. The shot will mean nothing for me, or it may mean everything.”
Those who don’t plan on getting a the third COVID-19 vaccine dose are worried about side effects and lack of information.
Despite ACR recommendations that people on immunosuppressive or immunomodulating medication get a third dose of the COVID-19 vaccine, some respondents said they don’t plan on doing so — or at least, not yet.
Of the 47 percent respondents who had not yet received a vaccine, 9 percent (110 people) said they don’t plan on getting one. Many shared their reasoning in a free response section.
Some expressed concerns about debilitating side effects and disease flares, based on how they reacted to the original vaccine series.
“I was sick for seven weeks after my second dose which started day after getting vaccine. I won’t chance it,” one patient wrote.
“The second shot seems to have created a flare up of my PMR (polymyalgia rheumatica),” one patient shared. “I’m afraid a third shot might make me even sicker.”
Another patient echoed this, writing, “I got a bad rheumatoid arthritis flareup four days after second vaccination and it has gotten worse. I’m afraid that the third vaccination would make arthritis even worse.”
Others, however, are simply “waiting for more information.”
“I plan to wait to learn of positives or negatives of the third shot,” one person shared.
Another patient wrote that there is, “not enough information on it,” to make them feel comfortable.
If you have questions or concerns about getting the third COVID-19 vaccine dose, talk to your health care provider.
The Global Healthy Living Foundation is committed to providing ongoing education about COVID-19 vaccines for the chronic illness and immunocompromised community.
To stay informed about the latest COVID-19 vaccine news for people who are immunocompromised, take immunosuppressant medications, or have autoimmune conditions, follow all of our COVID-19 vaccine coverage here.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety.
About the Patient Support Program Quick Poll
Members of our program have underlying health issues — such as inflammatory arthritis and other autoimmune conditions, heart disease, lung disease, diabetes, and more — that may increase their risk for COVID-19 complications. They are interested in understanding the best ways to stay safe during the pandemic and to be part of a community of people with similar concerns, questions, and fears.
We regularly poll members, who live in the U.S. as well as around the globe, about a variety of topics, including how the pandemic is affecting their lifestyle, mental health, chronic disease management, medication adherence, and more.
We use this information to inform the educational resources we provide and to inform other stakeholders — such as public health experts, policymakers, advocacy groups, health care professionals, and pharmaceutical companies — about chronic illness patients’ needs and concerns. You can participate in ongoing polls by joining the support program here.
COVID-19 Vaccine Clinical Guidance Summary for Patients with Rheumatic and Musculoskeletal Disease. American College of Rheumatology. August 19, 2021. https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf.
COVID-19 Vaccines for Moderately to Severely Immunocompromised People. U.S. Centers for Disease Control and Prevention. August 13, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
Haidar G. Immunogenicity of COVID-19 Vaccination in Immunocompromised Patients: An Observational, Prospective Cohort Study Interim Analysis. medRxiv. June 30, 2021. doi: https://doi.org/10.1101/2021.06.28.21259576.
Possible Side Effects After Getting a COVID-19 Vaccine. U.S. Centers for Disease Control and Prevention. August 6, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html.