If your doctor wants to know how you’re faring with a chronic condition like rheumatoid arthritis, they have a number of tools to assess this. They can look at your bloodwork for signs of inflammation or imaging like X-rays, MRIs, ultrasounds, or CT scans to look for damage to your joints or organs. They can feel your joints for signs of pain or swelling.
And — importantly — they can have you, the patient, report how you’re feeling and functioning. This last part is known in the research world as patient reported outcomes (PROs) research. It is becoming increasingly important in evaluating how patients are doing with their disease management and to decide whether treatments are working.
Doctors can have patients fill out paper questionnaires (say, in the waiting room) to assess PROs, but it’s becoming easier for patients to track this on their own using digital tools. ArthritisPower, for example, is a patient-centered research registry and application from our non-profit, The Global Healthy Living Foundation. It allows people with arthritis and musculoskeletal disease to select PROs they want to track — such as pain, fatigue, mental health, and more — and fill out surveys regularly on their smartphone or computer.
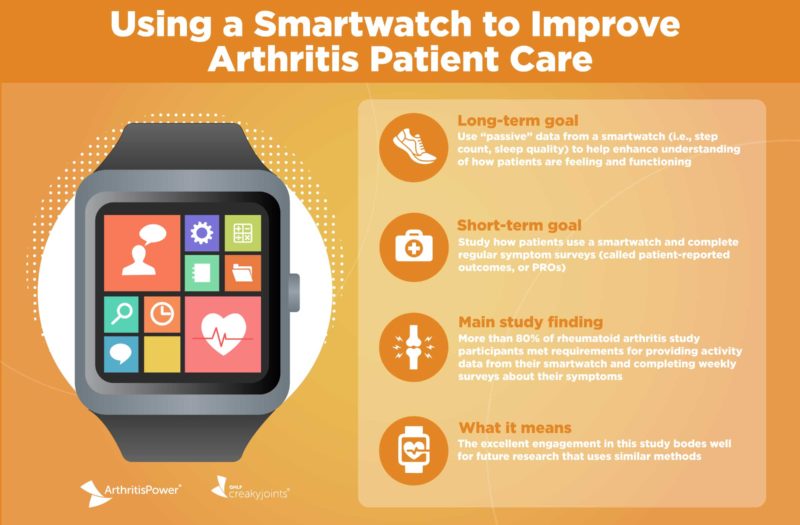
There’s also another form of patient-generated data that can work hand in hand with patients completing their own PROs — “passive” biometrics that can be collected from smartwatches or other similar tracking tools. (Think daily step counts, sleep quality, heart rate, and more.) They’re considered passive because they’re being collected in the background without you actively needing to document them yourself. And your doctor could use such data points to gauge how you’re doing in addition to PROs and clinical arthritis disease activity measures.
“Rheumatologists generally see their patients once every three or more months so it’s hard to know what the patient experiences during the interim,” explains study co-investigator Jeff Curtis, MD, rheumatologist and pharmacoepidemiologist at the University of Alabama at Birmingham School of Medicine. “Studies that use digital tools such as smartphones and smartwatches or other activity trackers can help shape a future where such tools are commonplace in rheumatology patient care and help enhance monitoring in between doctor visits.”
Studying the Feasibility of Gathering This Patient Data
The Global Healthy Living Foundation and our ArthritisPower research registry are committed to being on the forefront of innovation in patient-centered research. So naturally we want to study how both active and passive PRO data could work together to paint a more accurate picture of patients’ health.
In research presented during ACR Convergence 2020, the annual meeting of the American College of Rheumatology, we share the findings of an important new study that asked a group of people with rheumatoid arthritis to 1) wear a smartwatch and ensure that data from it is being captured on a regular basis, and to 2) report PROs about pain, fatigue, and more on a regular basis.
The goal of the research was to understand to what extent participants would be engaged with and “adherent” to the study. If the researchers could demonstrate that rheumatoid arthritis patients were responsive to filling out their PROs and wearing and syncing their smartwatch (in this case, a Fitbit® Versa™) regularly, they could then design future studies to help answer specific questions about how the addition of passive data could enhance our understanding of disease.
For example, could changes in patients’ daily step counts help predict when they might be entering an arthritis flare? Could a smartwatch’s sleep sensors objectively measure how well a new medication is addressing painsomnia?
What this initial research showed is that a thoughtful and well-executed study plan did indeed demonstrate that it is feasible to collect PROs and passive biometric data from patients at the same time. The study also revealed certain characteristics of patients who would be most likely to consistently participate.
How the ‘ArthritisPower Smartwatch Study’ Worked
The study had a “lead-in” and “main study” period. Initially, 470 rheumatoid arthritis patients expressed interest in participating and began the study lead-in. During this phase, participants had to complete for at least 10 days short daily PROs (two questions about pain and fatigue) and a longer set of PRO questions once a week.
It was almost like a tryout to make sure people wanted to participate in the longer study.
Lead-In Study
Participants who successfully completed the lead-in “tryout” were then mailed a smartwatch and entered the main study after they first synced their watch.
Main Study
Of the initial 470 patients, 278 finished the lead-in and began the main study. Here, patients had to fill out the same two daily PRO questions and weekly PRO questionnaire for 84 days (three months). They also had to wear their smartwatch at least 80 percent of each day and charge and sync it regularly.
A greater proportion of patients who progressed from the lead-in to the main study were employed (versus unemployed), took one biologic medication, and had lower daily pain and fatigue measures compared with those who did not qualify for the main study.
Researchers may be able to use information like this to predict which patients may be more engaged with research that involves digital data collection — and to help support patients who may have a hard time with study tasks, especially as they get started.
What We Learned About Patient Engagement
Overall, engagement among the patients in the main study was high.
- 87 percent of patients completed their weekly PRO surveys for at least 70 percent of the study duration
- 82 percent of patients provided their smartwatch data as directed at least 70 percent of the time
- 57 percent of patients completed their daily PRO questions at least 70 percent of the time
Overall, more than half (53 percent) of participants provided “composite data” — or fulfilling all three data points — 70 percent of the time. More than 80 percent of participants were adherent at providing activity and weekly PRO data.
“The fact that patient engagement in this study was as high as it was demonstrates the importance of using a patient-centered study design,” says study lead W. Benjamin Nowell, PhD, director of Patient-Centered Research at GHLF and ArthritisPower Principal Investigator. “For example, the lead-in period helped ensure that patients understand the research commitment and what it feels like to participate. And it’s encouraging that four out of five participants met the adherence requirements for providing activity data from their smartwatch and completing their weekly PROs. We are eager to apply what we learned from this research to future studies using passive data from smartwatches or other trackers to enhance our understanding of patients’ experiences with chronic pain and other symptoms.”
Found This Study Interesting? Get Involved
If you are diagnosed with arthritis or another musculoskeletal condition, we encourage you to participate in future studies by joining CreakyJoints’ patient research registry, ArthritisPower. ArthritisPower is the first-ever patient-led, patient-centered research registry for joint, bone, and inflammatory skin conditions. Learn more and sign up here.
This study was sponsored by Eli Lilly, which is a corporate sponsor of the Global Healthy Living Foundation.
Nowell W, et al. Participant Engagement and Adherence in an ArthritisPower Real-World Study to Capture Smartwatch and Patient-Reported Outcome Data Among Rheumatoid Arthritis Patients. [abstract]. Arthritis & Rheumatology. November 2020. https://acrabstracts.org/abstract/participant-engagement-and-adherence-in-an-arthritispower-real-world-study-to-capture-smartwatch-and-patient-reported-outcome-data-among-rheumatoid-arthritis-patients.