Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
Earlier this week, President Biden announced that all U.S. adults should be eligible for the COVID-19 vaccine by April 19, speeding up his original timeline by almost a month. Waiting to get the vaccine and actually getting it, however, can bring about an array of emotions. You may be excited and hopeful about a return to so-called “normalcy,” but you may also be anxious about various unknowns (say, variants) or worried about society reopening too quickly.
These emotions are intensified for our community of people with chronic illness, who continue to seek more information about how COVID-19 affects people with autoimmune diseases; what extra precautions, if any, should they take; and, more recently, whether or to what degree the COVID-19 vaccine might be less protective for people who take immunosuppressive medication (as do many people with autoimmune and inflammatory conditions.)
But even though there is so much unknown in general and for people with chronic illnesses in particular, many are still excited and hopeful about what being vaccinated can mean for their future.
A recent poll of 2,379 people, conducted by the Global Healthy Living Foundation (GHLF) as part of its COVID-19 Patient Support Program, aimed to gain insight into how people with chronic illnesses feel emotionally about the COVID-19 vaccine. The poll, which was conducted between April 1-4, 2021, specifically looked at the various emotions of patients who either were waiting to receive the vaccine and those who already received any dose of the COVID-19 vaccine. This means they could have received one or two doses of a two-dose vaccine (such as Pfizer or Moderna), or one dose of a single-dose vaccine (such as Johnson & Johnson).
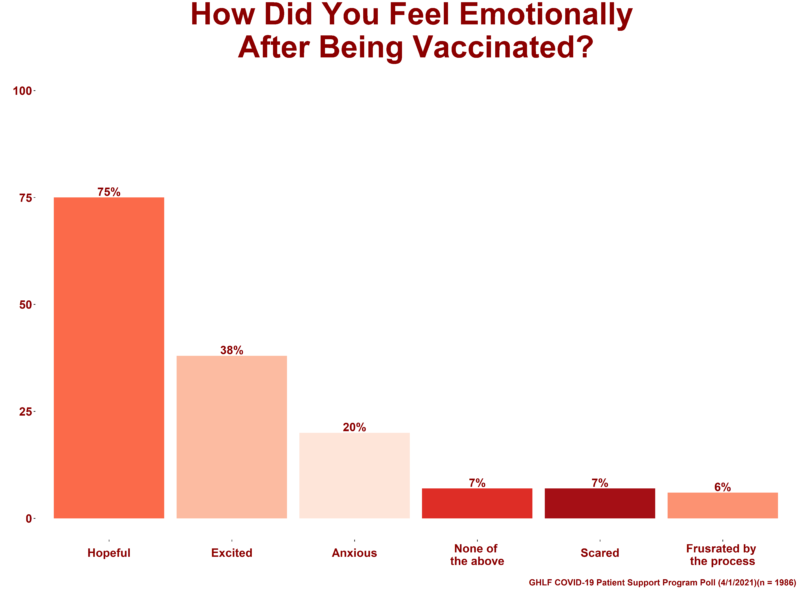
Of those who responded, 1,986 patients — about 84 percent — reported receiving some dose of the COVID-19 vaccine.
These patients were then asked to choose any and all emotions from a provided list that described how they felt after receiving the vaccine:
- 20% said they felt anxious
- 38% said they felt excited
- 6% said they felt frustrated by the process
- 7% said they felt scared
- 75% said they felt hopeful
- 7% said they felt none of the above
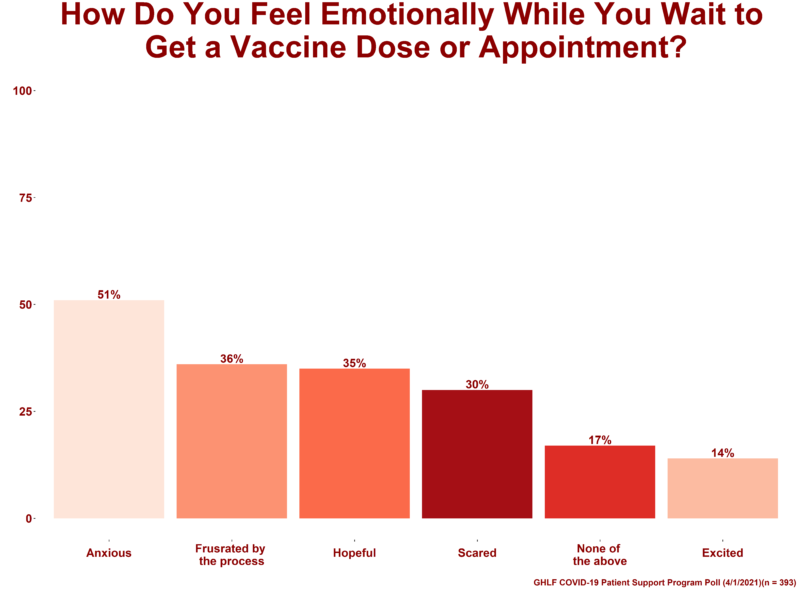
The 393 patients — about 16 percent — who reported not receiving any vaccine doses were also asked to choose any and all emotions from a provided list that described how they felt waiting to get the vaccine:
- 51% said they felt anxious
- 14% said they felt excited
- 36% said they felt frustrated by the process
- 30% said they felt scared
- 35% said they felt hopeful
- 17% said they felt none of the above
More than 2,300 respondents filled in the free-response section of the poll, in which they were able expand on their feelings.
Here are some insights into the emotions people with chronic illnesses feel about waiting for and getting the COVID-19 vaccine.
People who received any dose of the COVID-19 vaccine are excited and hopeful about their post-vaccine life.
For some, the positive outlook is tied to feelings of newfound safety and a return to “normal.”
“I am hopeful and excited to live my life without the fear of getting really sick from COVID if I am to contract it,” one person wrote. “I don’t want to have to ever go on a ventilator and fear that COVID would ruin my already weak kidneys and liver.”
Similarly, another individual wrote that they “felt excited and hopeful because [getting the vaccine] was the first step in getting protection from the virus, and suggests that a life of more freedom was going to come back again.”
“We’re both so excited for the world to become semi-normal,” one patient said of herself and her husband. “I’m a week into my second shot and feel like the whole world has opened back up to me, after being isolated for a year.”
“[I’m] hopeful that maybe we can start getting back to normal social interactions,” said a respondent who works in a hospital. “Just knowing I have some added protection against infection is [also] a huge relief.”
For others, the positive outlook comes from knowing they will be able to reunite with loved ones.
“[I’m] excited to have human contact with my loved ones who have been vaccinated, especially my 97-year-old mom [whose] [t]ime is limited,” one patient wrote.
“I’m excited to see my grandchildren again and to be able to hug them,” another person wrote. “I can now see my sister before she dies. . . We can help with our son’s family when our daughter-in-law has a kidney transplant.”
Some, however, will still be proceeding with caution around loved ones. One patient emphasized that they were excited to see “already vaccinated parents, family, and friends.” Another wrote, “I’m ready to wear masks while seeing other vaccinated family and friends.”
Many patients — whether they’ve received any doses of the vaccine or not — are anxious, likely due to limited research on how the vaccine effects immunocompromised people.
Although evidence shows that the Pfizer vaccine and Moderna vaccine have around 90 percent efficacy and the Johnson & Johnson vaccine had up to 85 percent efficacy against moderate to severe COVID-19, there is not yet data on the vaccine’s effectiveness in people on immunosuppressant medications.
This, understandably, has caused anxiety among those with autoimmune and inflammatory conditions.
“I’m excited to finally be vaccinated and able to do things, [but] anxious that it might impact me differently because of my disease and cause problems,” one respondent wrote.
“The experts only know how well the vaccine works and for how long it lasts in people [who are] not like me,” another wrote. “Everyone keeps talking about fully vaccinated people like we’re all in the same boat when we’re not.”
“With all my autoimmune issues I’m nervous and worried about how [the vaccine] will impact me because there are less studies in this area,” one patient wrote. “I’m also anxious to get it for those reasons.”
“[I’m] anxious and scared because of the unknowns, [as] I have reacted poorly to vaccines in the past due to my disease,” someone wrote, adding that they don’t know if they will get the vaccine as a result.
But it’s not just the unknowns around vaccine safety and effectiveness that make immunocompromised people anxious. Many wrote that the actions of others have them worried and scared.
“I’m anxious and scared because I see the numbers of people getting infected going up and the government not imposing enough restrictions to flatten the curve,” said one respondent who has not received any vaccine doses.
Similarly, another not-yet-vaccinated patient shared, “I am anxious about others getting the vaccine and not taking precautions anymore.”
“[I’m] worried people are going to stop wearing masks and social distancing, making it not only more of a risk for COVID to spread but other viruses too,” someone wrote.
Despite getting the vaccine, one patient wrote they are “still anxious about herd immunity [and] do not feel safe enough yet.”
Patient frustration stems from the rollout process, which many say is slow and confusing.
For many, knowing if they’re eligible for the vaccine and how to get the vaccine has caused a great deal of stress.
“It was horribly difficult to figure out how to get the vaccine,” one respondent wrote. “Nothing was made simple or user friendly. . . And not only did each state have its own rules but each county within the state had rules and requirements that differed greatly.”
Another person shared a particularly upsetting story about their vaccine registration experience.
“I applied to get a vaccination over a month ago if not longer,” they wrote. “They called to set up an appointment . . . When I said I have had allergic reactions to antibiotics, the women told me they recommend I get the Johnson & Johnson [vaccine] and that someone else will call me back. I have not heard from anyone.”
Editor’s note: Allergic reactions to antibiotics are not considered a contraindication to getting a COVID-19 vaccine, but if you have any concerns about your history of allergies, you should check with your health care provider.
Some patients expressed frustration with or concerns over making changes to their medication regimens. For a small number of immunosuppressant medications, including methotrexate, it may be recommended that you temporarily pause taking the medication after receiving the vaccine in order to help your body mount a stronger immune response. In some cases, this means deciding if the risks of forgoing disease-modifying medications — such as worsening symptoms or a risk of a disease flare — are worth the “reward” of possibly increasing the effectiveness of the COVID-19 vaccine.
“Suspending my regular schedule of medications the week after a shot makes me feel even more vulnerable,” one patient wrote.
“The system does not consider special timing for us,” one patient, who uses rituximab, wrote. “I just received one infusion, so I am frustrated that I cannot receive my first [vaccine] dose for the next five months.”
For more information on modifying medications for certain chronic illnesses before or after getting the vaccine, read the following resources:
- Psoriatic Arthritis and the COVID-19 Vaccine: What You Need to Know
- Rheumatoid Arthritis and the COVID-19 Vaccine: What You Need to Know
- Axial Spondyloarthritis and the COVID-19 Vaccine: What You Need to Know
But despite the worries and frustrations, many people are still hopeful.
Patients who are waiting for the vaccine do feel hopeful; for protection from COVID, for more control over the pandemic, and for a little more calm after more than a year of chaos.
“I’m excited and hopeful for myself and our community,” one patient wrote. “I am hopeful that with the vaccine our country can start to heal.”
The Global Healthy Living Foundation is committed to providing ongoing education about COVID-19 vaccines for the chronic illness and immunocompromised community.
To stay informed about the latest COVID-19 vaccine news for people who are immunocompromised, take immunosuppressant medications, or have autoimmune conditions, follow all of our COVID-19 vaccine coverage here.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety.
About the Patient Support Program Quick Poll
Members of our program have underlying health issues — such as inflammatory arthritis and other autoimmune conditions, heart disease, lung disease, diabetes, and more — that may increase their risk for COVID-19 complications. They are interested in understanding the best ways to stay safe during the pandemic and to be part of a community of people with similar concerns, questions, and fears.
We regularly poll members, who live in the U.S. as well as around the globe, about a variety of topics, including how the pandemic is affecting their lifestyle, mental health, chronic disease management, medication adherence, and more.
We use this information to inform the educational resources we provide and to inform other stakeholders — such as public health experts, policymakers, advocacy groups, health care professionals, and pharmaceutical companies — about chronic illness patients’ needs and concerns. You can participate in ongoing polls by joining the support program here.
Thomas K. Biden Says All U.S. Adults Should Be Eligible for Covid-19 Vaccine by April 19. The Wall Street Journal. April 7, 2021. https://www.wsj.com/articles/biden-to-say-all-u-s-adults-should-be-eligible-to-receive-covid-19-vaccine-by-april-19-11617711281.
Thompson MG, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. U.S. Centers for Disease Control and Prevention. April 2, 2021. doi: http://dx.doi.org/10.15585/mmwr.mm7013e3.
Vaccines and Related Biological Products Advisory Committee Meeting. Janssen Biotech, Inc. February 26, 2021. https://www.fda.gov/media/146219/download.