Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
If you’ve already received a third COVID-19 vaccine dose as part of your original vaccination series, you may now be eligible to receive a fourth dose as a booster.
This applies to people who are immunocompromised because of underlying medical conditions or immunosuppressant medications.
Of course, in the era of COVID-19, it can be confusing to keep track of “additional doses” and “booster shots” — and what they mean for you. Here’s everything you need to know about the fourth dose if you are immunocompromised.
Is the Fourth Dose Considered a ‘Booster’ for the Immunocompromised?
Yes. Originally, the U.S. Centers for Disease Control (CDC) provided guidance stating that immunocompromised individuals could receive a booster shot (a fourth dose) six months after their original three-dose series. Now, in an effort to curb the surge of infections from the highly infectious Omicron virus, the CDC has shortened that timeline to five months.
That means if you received a third mRNA (Pfizer or Moderna) vaccine dose when it was first authorized for immunocompromised individuals in August 2021, it will have been five months this January 2022.
“There is no current American College of Rheumatology guidance on [the fourth dose], but it is a fast-moving issue,” says Jeffrey Sparks, MD, MMSc, Assistant Professor of Medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston. “I suspect they will offer similar guidance like the CDC soon.”
Keep in mind that a third dose of an mRNA (Pfizer or Moderna) shot for those with moderately or severely compromised immune systems is considered part of their original series. Immunocompromised individuals may need this additional shot as part of their primary series to mount a better immune response. A fourth shot or booster shot, on the other hand, is meant to enhance protection after it has started to wane over time, per the CDC.
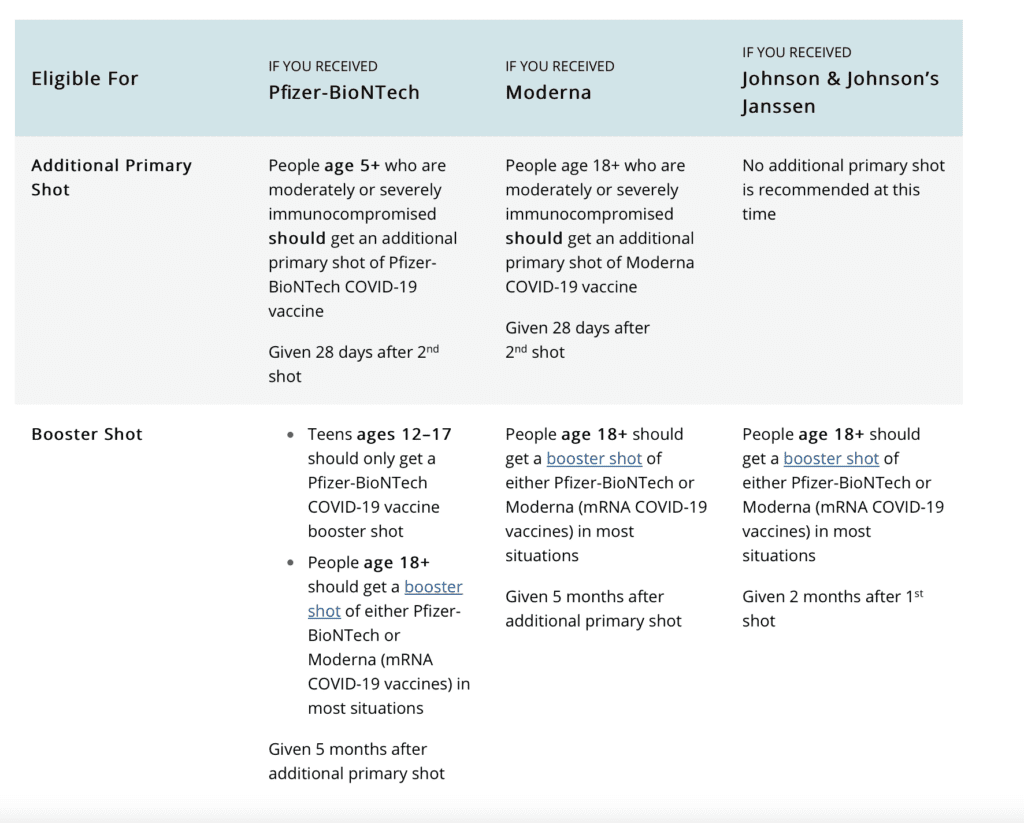
People aged 18 and older who received the Johnson & Johnson vaccine should get a booster shot of either Pfizer-BioNTech or Moderna in most situations — two months after the first shot. The guidance does not yet recommend an additional booster for this group, but this may change as more data emerges.
Does the Fourth Dose Need to Be the Same Type as Your First Three Doses?
The CDC says the mRNA vaccine used for the additional primary shot (third dose) should be the same as the one used for the primary vaccine series. However, if the mRNA vaccine given for the first doses is not available or is unknown, either mRNA COVID-19 vaccine product can be administered for the third dose or the fourth booster dose.
“Per available data, it’s safe to mix and match,” says Jean Liew, MD, Assistant Professor of Medicine at Boston University School of Medicine. “The data I’ve seen have not demonstrated that any combination is better than another. The only exception is that if you got a non-mRNA vaccine like Johnson & Johnson before, it’s better to get an mRNA vaccine now.”
For the Moderna vaccine, the fourth booster shot will be half the dose from the primary series. The 0.5 mL (100 micrograms) dosage is used for all three doses of the primary series for immunocompromised patients to mount a sufficient initial immune response, but the lower booster dose of 0.25 mL (50 micrograms) is recommended for boosters based on clinical trial data that showed this lower dose was safe and effective, per the CDC.
How Well Might a Fourth Dose Protect Against Omicron?
In the general population, it appears that booster doses — which are third doses — protect against severe disease when it comes to the very transmissible variant Omicron.
“Vaccines do not necessarily prevent infection, but should make them milder,” says Dr. Sparks. “It appears this is the case with Omicron. It is very possible that even boosted patients may experience infection, but they are much less likely to require hospital admission or experience other poor outcomes.”
However, it gets more complicated when it comes to the immunocompromised population.
“If you have generated any antibody responses in the past, the fourth dose is likely to generate protective antibodies against Omicron,” says researcher Alfred Kim, MD, Assistant Professor of Medicine, Pathology, and Immunology at Washington University. “But if you haven’t made any antibodies in the past three doses, the chances are much lower that protective antibodies will be made with the fourth dose.”
In other words: If you haven’t already produced antibodies with previous shots, you might not now, either — which could leave you more susceptible to Omicron. However, this isn’t a hard and fast rule, and some early research is promising.
“There’s some data in solid organ transplant patients on fourth doses,” Dr. Kim told CreakyJoints previously. “Most importantly, it remains safe. In organ transplant cases where immunosuppression is substantially burdensome — they have some of the highest, most aggressive immunosuppressive regimens out there — there are increasing numbers of people starting to respond to fourth doses, even though they did not respond to the first two or three doses.”
Plus, antibodies aren’t the only measure of immune protection, which is why it’s important to still get the fourth vaccine dose. Experts are working to determine if other parts of the immune system, such as T cells, can provide protection in the absence of protective antibodies.
“It appears that most patients make a T cell response, even if they do not make detectable antibodies,” says Dr. Sparks. “We hope this translates to some protection from vaccines for individuals who did not have an antibody response.”
For all these reasons, your doctor will likely recommend the fourth dose if you are immunocompromised.
“The data is starting to emerge that getting a fourth dose is probably going to be part of what we look forward to in 2022 for many of our immunocompromised patients,” Dr. Kim told us previously.
Read more about Omicron and the immunocompromised in this guide for those at high risk for COVID-19.
Who Is Eligible for a Fourth ‘Booster’ Dose?
The CDC considers the following groups of people moderately to severely immunocompromised and eligible for a fourth dose:
- Been receiving active cancer treatment for tumors or cancers of the blood
- Received an organ transplant and are taking medicine to suppress the immune system
- Received a stem cell transplant within the last 2 years or are taking medicine to suppress the immune system
- Moderate or severe primary immunodeficiency (such as DiGeorge syndrome, Wiskott-Aldrich syndrome)
- Advanced or untreated HIV infection
- Active treatment with high-dose corticosteroids or other drugs that may suppress your immune response
The ideal COVID-19 vaccine plan for these individuals is as follows:
- Get a primary vaccine series: 2 doses of Pfizer or Moderna, followed by a 3rd dose 28 days later
- Get a 4th booster dose 5 months after the 3rd dose
For those who originally received the Johnson & Johnson vaccine:
- Get a booster of Pfizer or Moderna 2 months after 1st dose
- No additional boosters (3rd doses) are recommended at this time
However, some people are trying to get the fourth dose even if they’re not eligible. Many people with compromised immune systems have sidestepped government guidance and received unauthorized fourth or fifth shots by either not disclosing previous or convincing pharmacists that they need an additional dose because of their lack of antibodies, per the New York Times.
Although the Food and Drug Administration (FDA) and CDC determine when additional doses should be administered, some patients and their doctors believe the agencies have acted too slowly to protect the most vulnerable.
Still, skirting the rules isn’t recommended. “Anything outside of the CDC’s recommendation should be discussed with your doctor,” says Dr. Sparks. “The risks are unknown since very few people have received vaccines outside of the regular schedule. There are some clinical trials ongoing studying this, but we do not currently know the results.”
Plus, there are other, approved ways to protect yourself from COVID-19. The FDA recently authorized a preventive monoclonal antibody treatment, Evusheld, for the immunocompromised. This preventive treatment is administered before exposure or infection to COVID-19. In one primary analysis, recipients saw a 77 percent reduced risk of developing COVID-19 compared to those who received a placebo. Here’s more about Evusheld for the immunocompromised.
When you do go to get your (approved) fourth dose, be prepared with your vaccine card and a list of medications to show that you’re eligible.
“Most places administering boosters are now requiring proof of prior vaccination, so bring vaccination cards,” says Dr. Kim. “As for proof of immunosuppression, this continues to be a verbal assurance of immunosuppressive medication use, so know your medications, dose, and frequency of dosing.”
If you’re concerned that you’ll be turned away, Dr. Liew recommends bringing a letter from your doctor. Still, experts are hopeful that it will be easy for eligible immunocompromised patients to get the booster shot.
“Fourth doses for immunocompromised individuals are currently recommended by the CDC and I am hopeful that this would be obtained as easily as a third dose, without need for special documentation,” says Dr. Sparks.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety.
COVID-19 Vaccines for Moderately or Severely Immunocompromised People. COVID-19. U.S. Centers for Disease Control and Prevention. January 7, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
Interview with Alfred Kim, MD, Assistant Professor of Medicine, Pathology, and Immunology at Washington University
Interview with Jean Liew, MD, Assistant Professor of Medicine at Boston University School of Medicine
Interview with Jeffrey Sparks, MD, MMSc, Assistant Professor of Medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston
Moderna COVID-19 Vaccine Questions. Vaccines & Immunizations. U.S. Centers for Disease Control and Prevention. December 6, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/moderna-faqs.html.
Mandavilli A. Starting Later This week, Some At-Risk Americans Become Eligible for a 4th Shot. The New York Times. January 9, 2022. https://www.nytimes.com/2022/01/09/health/immunocompromised-fourth-dose-booster.html.