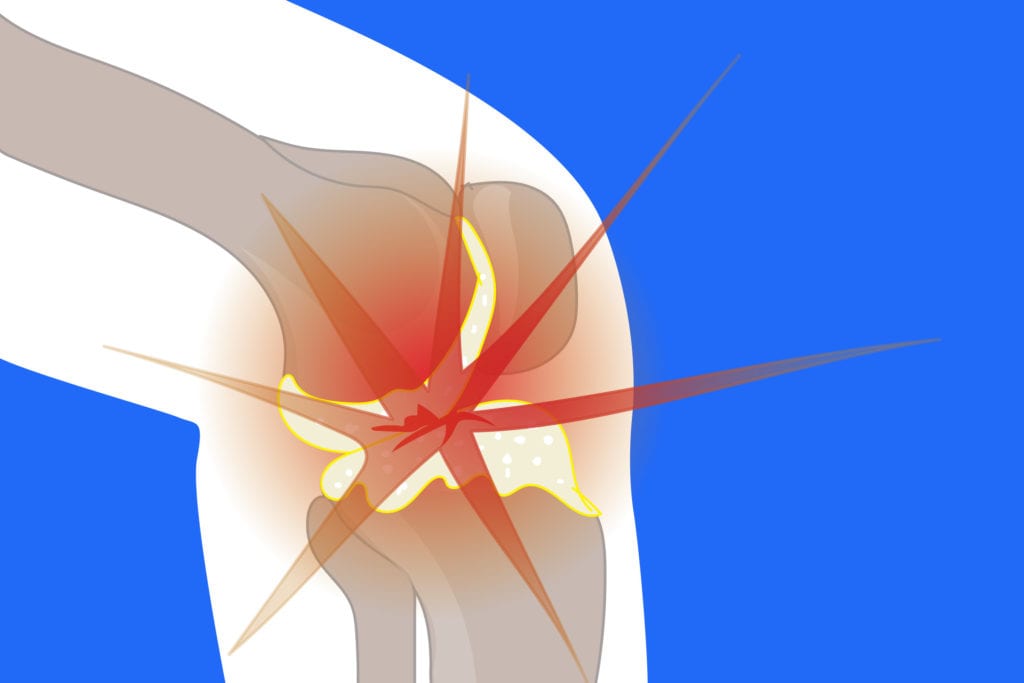

Despite promising results from an earlier trial, lutikizumab — a drug that blocks proteins tied to inflammation — did not hold up in a phase II trial of patients with knee osteoarthritis (OA).
The earlier study, a phase I trial published in 2017, found that people who received lutikizumab injections had decreased markers of inflammation in their blood, but that study included only 36 patients and lasted three months. The new study, which was published in the journal Arthritis and Rheumatology this month, included 350 patients and lasted a full year.
People in the new study were randomly selected to get one of three doses of lutikizumab or a placebo every two weeks for 52 weeks.
At the 16-week mark, the pain scores among people who had been getting the highest (100 mg) dose of the drug improved compared to those getting lower doses or a placebo. But by the end of the trial, there were no significant differences in symptoms or joint inflammation (as measured by MRIs or ultrasounds).
Also concerning: People who got lutikizumab dropped out of the study more often compared to those who received a placebo after they experienced injection site reactions and/or neutropenia (low count of a type of white blood cell, which puts you at risk for infections).
The authors concluded that the targeting interleukin-1 “is not an effective analgesic/anti-inflammatory therapy in most patients with knee OA and associated synovitis,” which is inflammation in the membrane lining the joints.
These findings come on the heels of other recent research on lutikizumab, which found that it did not help patients with hand OA, either.





